Radiometric dating methods
Table of half-life periods for some common radiometric isotopes
Parent Isotope ————> Daughter Isotope ————Half-Life
Carbon 14 —————-> Nitrogen 14 —————– 5730 years
Uranium 235 ————–> Lead 207 ——————- 704 million years
Potassium 40 ————-> Argon 40 ——————- 1.3 billion years
Uranium 238 ————-> Lead 206 ——————- 4.5 billion years
(note Carbon 14 is only useful for dating materials derived from living organisms less than about 60,000 years old)
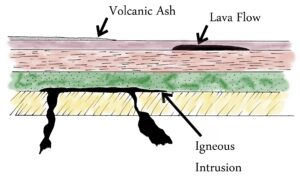
Sediments can be difficult to date by radioisotope methods. Igneous rocks can be aged more reliably because of the consistent composition of magma from which it forms. It can be concluded that sedimentary rocks found beneath lava or volcanic ash layers were present when the lava of ash was deposited and are therefore known to be older than those igneous layers. Likewise sedimentary rock surrounding an intrusion of magma are older than the intrusion.
Using igneous formations to estimate the ages of sedimentary beds